Illuminating the life of a cell
By tagging molecules in a cell with fluorescent labels that switch on and off, MIT engineers can study their interaction to learn more about how cells operate.
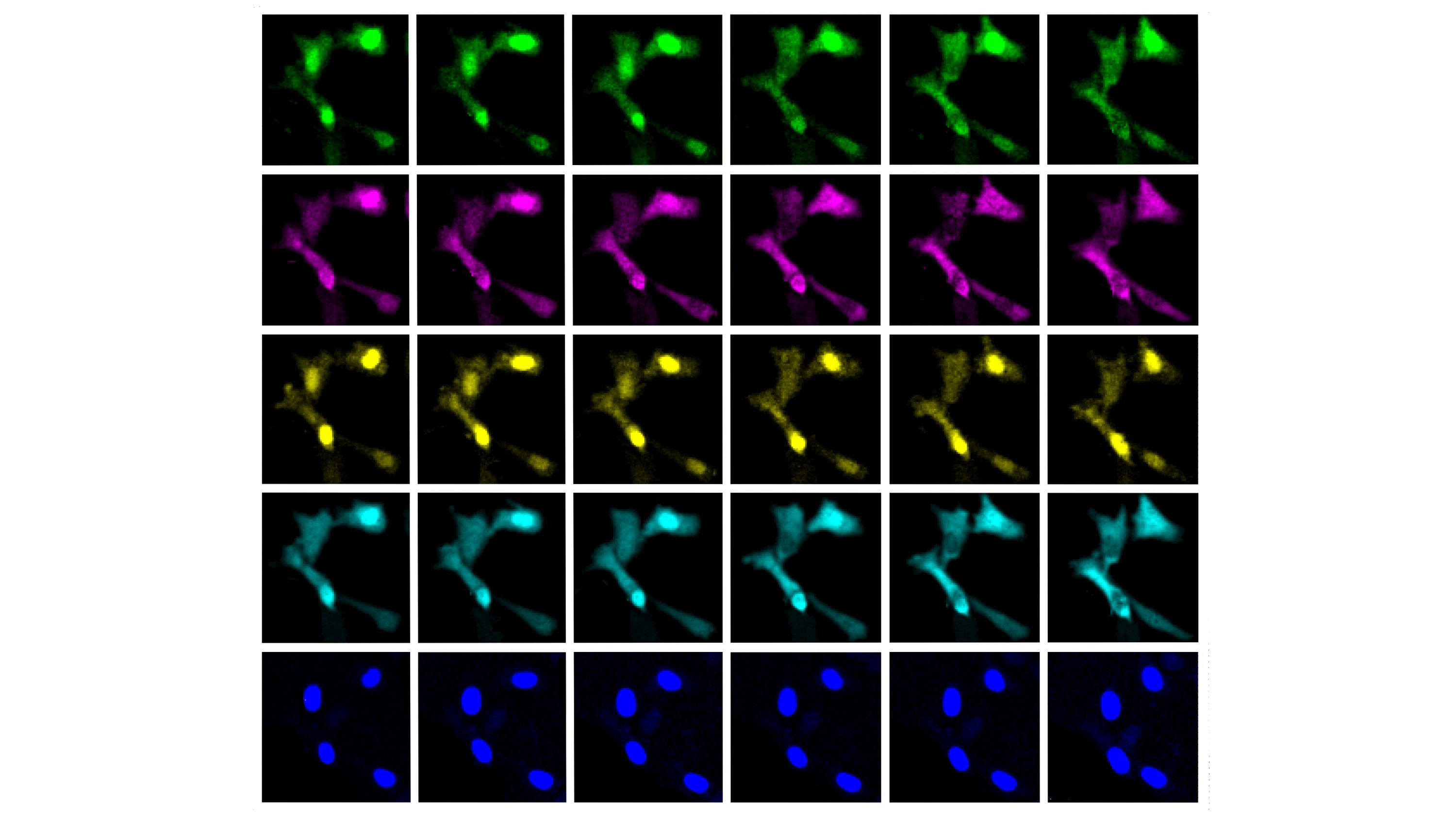
Living cells are bombarded with molecular signals that influence their behavior. Being able to measure those signals and the response to them could help scientists learn much more about how cells work, including what happens as they age or become diseased.
Labeling molecules inside cells with fluorescent proteins that glow in different colors has been a promising approach, but a typical microscope can distinguish only two or three of these colors. Now MIT researchers have found a way to observe at least twice that number, making it possible to watch the signals in a cell in real time.
“There are many examples in biology where an event triggers a long downstream cascade of events, which then causes a specific cellular function,” says Edward Boyden ’99, MEng ’99, a professor of biological engineering and of brain and cognitive sciences at MIT, who is the senior author of a paper on the work. “We wondered, could you simply watch it happen?”
In 2020, Boyden’s lab developed a way to simultaneously image up to five different molecules within a cell by targeting the fluorescent tags to distinct locations. This approach, known as “spatial multiplexing,” allows researchers to distinguish signals for different molecules even though they may all glow in the same color.
In the new study, the researchers took a different approach: instead of distinguishing signals by their physical location, they created signals that vary over time. The technique relies on “switchable fluorophores”—green and red fluorescent proteins that flicker on and off at different rates.
Each fluorophore can be used to label a different type of molecule within a living cell, such as an enzyme, a signaling protein, or part of the cell cytoskeleton. After imaging the cell for minutes, hours, or even days, the researchers use a computational algorithm known as linear unmixing to pick out the specific signal from each fluorophore, analogous to how the human ear can pick out different frequencies of sound.
Once this analysis is complete, the researchers can see when and where each labeled molecule was found in the cell during the imaging period. The imaging itself can be done with an ordinary microscope.
Using mammalian cells grown in a lab dish, the researchers demonstrated their approach by labeling six molecules involved in the cell division cycle. They also showed that they could label various enzymes, cell structures, and organelles such as the cytoskeleton and mitochondria.
This method could be useful for observing how cells respond to any kind of input, such as nutrients, immune system factors, hormones, or neurotransmitters. It could also be used to study how cells respond to changes in gene expression or genetic mutations. All these factors play important roles in growth, aging, cancer, neurodegeneration, and memory formation.
“You could consider all of these phenomena to represent a general class of biological problem, where some short-term event—like eating a nutrient, learning something, or getting an infection—generates a long-term change,” says Boyden, who is also a Howard Hughes Medical Institute investigator.
The researchers are now working on expanding the repertoire of switchable fluorophores and hope to adapt the system so that it could be used in mouse models.
Keep Reading
Most Popular
Large language models can do jaw-dropping things. But nobody knows exactly why.
And that's a problem. Figuring it out is one of the biggest scientific puzzles of our time and a crucial step towards controlling more powerful future models.
OpenAI teases an amazing new generative video model called Sora
The firm is sharing Sora with a small group of safety testers but the rest of us will have to wait to learn more.
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
Google DeepMind’s new generative model makes Super Mario–like games from scratch
Genie learns how to control games by watching hours and hours of video. It could help train next-gen robots too.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.